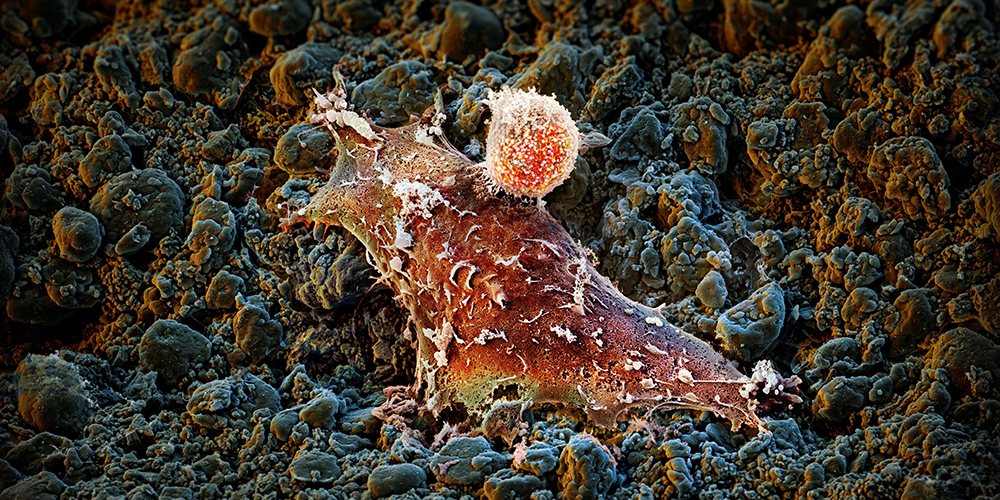
Therapy 2.0.
Text: Yvonne Vahlensieck
For some tumors, immunotherapy is an excellent treatment option, and for others, it is not as effective. Basel researchers are working on ways to make all cancer cells vulnerable to attack.
About ten years ago, a new generation of cancer treatments sparked great hopes: The idea was that the immune system – which is naturally designed to defend our body against dangerous invaders – is also capable of destroying cancer cells.
The initial hype was not entirely without justification. “Immunotherapy has since become a standard method of treating cancer-related illnesses; I'd estimate that over 40 percent of our patients receive treatments of this kind,” says Heinz Läubli, who heads a research group in the field of cellular cancer immunotherapy at the University of Basel and serves as senior physician in University Hospital Basel's Division of Oncology.
“Cancer immunotherapy” is an umbrella term for numerous methods that aim to activate the immune system to combat malignant cells. Often, this involves the use of “checkpoint inhibitors”. This approach is based on the realization that tumor cells use natural inhibitory pathways in the immune system, known as checkpoints, for their own purposes: namely, to defend themselves from attacks by immune cells.
Cancer cells activate these pathways by presenting certain structures on their cell membranes. Drugs known as checkpoint inhibitors neutralize this mechanism, for instance, by blocking the corresponding checkpoint structures on the cancer cells. This prevents the immune system's inhibitory response from being activated so that it can attack the tumor cells at full force.
Another approach is cellular immunotherapy: Here, for example, certain white blood cells, known as T cells, are filtered out of the cancer patient's blood and genetically modified so as to improve their ability to identify cancer cells when they are returned to the patient's body. One of these methods, CAR T cell therapy, is particularly effective against blood cancer.
Tumors deceive the immune system
“Over the past few years, new immunotherapies have paved the way for substantial progress that has helped many people,” says Läubli. That is especially true of cancers such as lung cancer or melanomas (skin cancer) in advanced stages, which previously had very low survival rates. “The data show that, in the case of malignant lung cancer, half of patients treated with immunotherapy were still alive six years following treatment,” reports Läubli. Pharmaceutical companies have caught on, and are constantly launching new products. In the past three years alone, US agencies have approved over fifty new cancer immunotherapies, many of which are designed to be used in combination with established chemotherapies.
“At the same time, some of the shine has worn off,” says Alfred Zippelius, joint head of the University Hospital's Division of Oncology, who also supervises a research group in the Department of Biomedicine. The fact of the matter is that the new therapies offer little benefit to patients in two thirds of cases. On one end of the spectrum, there are “hot” tumors, which respond very well to immunotherapy; but on the other end, there are “cold” tumors, against which the immune system is defenseless. In the past few years, cancers researchers like Zippelius and Läubli have discovered the reasons why. To do this, they conduct experiments using both human tumor tissue and mice.
We now know that the effectiveness of an immunotherapy is not related to the type of cancer but depends instead on the individual properties of each tumor. Cancer cells that closely resemble normal body cells in outward appearance are particularly well protected, for instance. In other cases, the tumors simply bolt the doors; they prevent the cells of the immune system from penetrating their inner tissues in the first place. Yet it is now possible to isolate the white blood cells that successfully infiltrate the tumor in the cancer tissue, reproduce them in the lab, and then inject them back into the patient's blood. This improves the effectiveness of the therapy. For that reason, this method is currently being studied in clinical trials at the University Hospital Basel.
Microenvironmental considerations
In recent years, Zippelius's research group has demonstrated that the tumor's microenvironment also plays a role in determining whether or not an immunotherapy works. “Microenvironment” refers to the community of different cell types and signaling molecules located in direct proximity to the cancer cells. “We want to understand which factors need to converge before the immunotherapy can successfully fight off the cancer,” says Zippelius.
For example, in a recently published study, his team demonstrated that a particular signaling molecule helps attract the right immune cells inside the tumor. However, this is just one of hundreds of factors involved. According to Zippelius, it is difficult to manipulate the tumor's microenvironment precisely without a better understanding of its mechanistic interactions: “If we don't face this complexity, we'll never succeed.”
A protective coat of sugar
Läubli, too, is exploring new frontiers with his team. Until now, immunotherapy was largely focused on proteins attached to the surface of the tumor cells. Yet cancer cells are also studded with scores of sugar chains, and it has been found that these molecules form a kind of protective sheath to defend against attacks by the immune system. In their trials, Läubli's research team made precise cuts in these sugar chains, and as a result, the immune cells were able to attack the cancer more effectively.
Aside from the “cold” tumors, there is yet another major issue with cancer immunotherapy: After a few weeks or months, T cells become exhausted and are no longer able to destroy the cancer cells. The two research groups at the University of Basel have now developed a method to study this phenomenon. They have identified a gene in the T cells that is responsible for causing this state of exhaustion. Without this gene, the T cells remain healthy indefinitely. One possible therapeutic approach in cellular immunotherapy, therefore, would be to switch off this gene in the isolated T cells before returning them to the patient's bloodstream.
Zippelius believes that the future of cancer treatment lies in finding the right combination of different therapies – ideally, precisely tailored to the properties of the individual tumor. But currently, this goal is still just out of reach. “After all these years, we still haven't come close to harnessing the full potential of cancer immunotherapy,” says Zippelius. “We're going to have to continue making major investments in research so that we can keep reaping the benefits of these therapies.”
More articles in this issue of UNI NOVA (May 2023).