How bacteria reinforce their protective shield
Researchers at the University of Basel have discovered a new mechanism by which bacteria ensure that their outer cell membrane remains intact and functional even under hostile conditions. This mechanism is important for the pathogen’s survival in the host. The study provides new insights underlying pathogenic virulence.
22 October 2020
When infecting a host, pathogens not only need to evade the immune defense but also to survive diverse living conditions, such as the acidic conditions in the stomach or the salty intestinal environment. Gram negative bacteria, many of which are pathogens, protect themselves from harmful external influences by an additional outer membrane.
In order to survive unfavorable conditions, bacteria must be able to continuously replace damaged outer membrane proteins and adapt their arsenal of proteins to the specific needs. This is the job of the bacterial protein Skp. Based on its atomic structure, the researchers led by Professor Sebastian Hiller from the Biozentrum of the University of Basel have now uncovered how it modulates its activity. Using Salmonella as a model, their collaboration partner Professor Dirk Bumann has also demonstrated that Skp is important for the virulence and survival of pathogens in hostile host environments.
Shuttle service for proteins
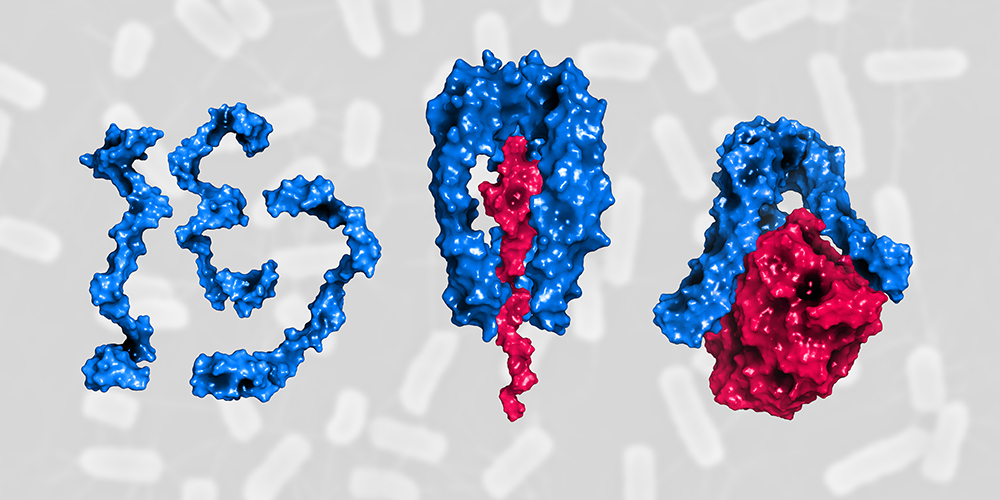
“Skp provides a kind of shuttle service in the bacterial cell. It protects and transports newly synthesized, still unfolded membrane proteins from the inner to the outer membrane,” explains Hiller. Without the help of Skp these proteins would form insoluble aggregates and accumulate in the intervening aqueous layer. As the turnover of membrane proteins can increase suddenly and rapidly, for instance under challenging conditions and cell stress, sufficient Skp shuttles must always be ready to meet the demand.
How this is achieved and how Skp regulates its own activity, has now been demonstrated by the researchers for the first time. “In the periplasmic space there is a large amount of individual Skp proteins that are disordered and therefore inactive,” says Guillaume Mas, first author of the study. “Under stress conditions, many Skp proteins are quickly recruited from this pool. They become active when three proteins assemble into a stable structure around the protein to be transported. Skp can carry out its shuttle function only in this state.” Upon the release of its cargo at the destination, the trimer dissociates and the individual Skp proteins are now available again for the next shuttle.
Protein function affects survival rate
During times of stress-induced high transport volumes of outer membrane proteins, the equilibrium of inactive single molecules shifts towards active trimers. This tightly controlled activation is essential for bacterial survival, protecting the cells from damage by preventing the accumulation of insoluble protein aggregates under stress conditions.
Salmonella with disturbed Skp function are at a disadvantage, as was shown in an animal infection model. Mutations in the protein affecting proper trimer formation reduce both the growth and survival rate of the bacteria in the host. These findings elucidating the role of the outer membrane in bacterial resistance are important for the treatment of infectious diseases.
Original publication
Guillaume Mas, Björn M. Burmann, Timothy Sharpe, Beatrice Claudi, Dirk Bumann, Sebastian Hiller
Regulation of chaperone function by coupled folding and oligomerization
Science Advances (2020), doi: 10.1126/sciadv.abc5822
Further information
- Prof. Dr. Sebastian Hiller, University of Basel, Biozentrum, tel. +41 61 207 20 82, email: sebastian.hiller@unibas.ch
- Dr. Katrin Bühler, University of Basel, Biozentrum, Communications, tel. +41 61 207 09 74, email: katrin.buehler@unibas.ch