Cell type atlas is an eye-opener
Text: Samuel Schlaefli
For the first time ever, researchers at the University of Basel have been able to show in detail that retinal tissue grown from induced pluripotent stem cells provides a suitable model for drug research. Key to this breakthrough was close collaboration between the Institute of Molecular and Clinical Ophthalmology Basel and University Hospital Basel.
Hereditary eye conditions such as Stargardt disease or retinitis pigmentosa were long considered incurable blinding diseases. Both diseases affect the retina, a very thin layer of nerve tissue at the back of the eyeball whose millions of lightsensing cells convert light into electrical impulses that are sent to the brain, where we perceive them as vision. Hereditary retinal diseases often emerge in childhood or adolescence and can, in the worst cases, lead to total blindness.
Lack of experimental models
Research into the causes of such hereditary eye conditions has remained challenging, as has the development of effective therapies. “In the life sciences, mice are a standard model for studying eye disease, but they have many limitations,” explains Dr. Cameron Cowan, senior researcher at the Institute of Molecular and Clinical Ophthalmology Basel (IOB). One reason is that mice lack a fovea, the central portion of the retina that is responsible for human high-acuity vision and is affected first in many eye conditions. Alternatively, retinal tissue donated from post-mortem patients would be a preferred specimen for laboratory testing. However, such tissue is hard to come by, with only about 120 potentially suitable donations made in Switzerland each year, while slow collection procedures leave the available tissue unhealthy.
Against this background, researchers have been working on a new model for several years now: Human cells obtained from various types of tissue, including blood, hair roots and skin, can be reprogrammed in a laboratory to grow into the desired organs, so-called organoids. This method is based on the trailblazing research of Japanese scientist Shin’ya Yamanaka, who was the first to successfully produce induced pluripotent stem cells in a laboratory. This won him the 2012 Nobel Prize in Medicine. Retinal tissue, too, can be produced by reprogramming stem cells. “Until now, the use of retinal organoids has been plagued with a great deal of uncertainty,” Cowan explains. “We had no way of knowing whether they would develop as in humans, or under what circumstances this retinal tissue could provide a suitable model.”
To find an answer, it was necessary to characterize the human retina in greater depth than ever before. Success, here, depends above all on the quality of the tissue used. Tissue from donor organs, however, deteriorates rapidly after the operation even if oxygen is artificially supplied. “We were able to reduce the time the tissue spends without oxygen from an average of eight hours to five minutes,” says Cowan. “This would not have been possible without close collaboration with the clinicians at the hospital.”
Establishing tissue quality
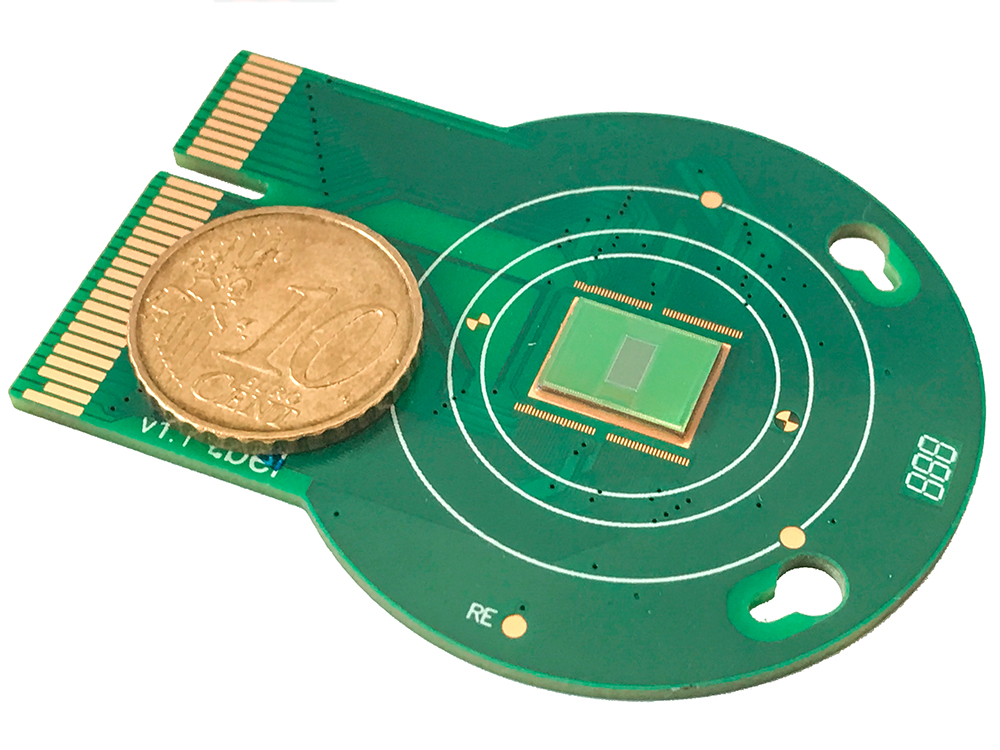
Assessing the condition of this unique tissue required a further innovation: Colleagues at the Department of Biosystems Science and Engineering at ETH Zurich developed a special microelectrode array, just a few square millimeters in size but packed with 26,000 electrodes, to measure cell function. In this process, ganglion cells in the retina are artificially oxygenated and placed adjacent to the electrodes. The photoreceptors are then stimulated by light, which they convert into electrical signals that are transmitted to the retinal ganglion cells. Electrical impulses from ganglion cells, intended for the visual centers of the brain, are intercepted by the electrodes. “To replicate the retinal input in the laboratory, we miniaturize the output of a video projector so that the image size corresponds exactly to that of the retina,” says Cowan. The researchers were able to measure the responses of thousands of retinal cells with the aid of the microelectrode array and confirmed the results through repeated light stimulation. “Nobody had ever measured such activity from post-mortem human retinal tissue,” he says with pride.
Based on tissue proven to be of high quality, the team of researchers has created a cell-type-specific “Gene Expression Atlas” for the human retina. This involved sequencing the RNA of approximately 100,000 single cells from different regions of the retina and assigning genetic “barcodes” to them. For the first time ever, the cell-type-specific transcriptome of the healthy human retina has been made possible, i.e. the sequencing of the RNA contained in its cells. This is relevant because the RNA is responsible for gene expression, in other words, triggering specific genetic programs. What was largely unknown was the types of retinal cells in which many disease-associated genes are expressed, and under what circumstances and from what stage this expression is paralleled in organoid cells.
Using their Gene Expression Atlas for the healthy human retina, the scientists are now able to precisely characterize the cell tissue of the retinal organoids grown in the laboratory. This was achieved by measuring the gene expression of approximately 50,000 organoid cells at six points in time over their 38-week growth period. “We were able to demonstrate that our lab-grown retinal organoids developed fully ex vivo and that they developed in much the same way as in humans and with the same gene expression patterns.” The correspondence was found to extend to the cell-type-specific expression of genes linked to particular eye conditions.
Paving the way for personalized medicine
Cowan is convinced that this model holds enormous potential for the development of new treatment approaches: “In the past, we’d often make assumptions about which cell types expressed the genes responsible for a particular disease. Today, we’re able to pinpoint them through gene expression analysis.” By way of example, he adds that assumptions made in developing new gene therapies for Stargardt disease about the distribution of mutated genes in tissue have now been shown to be inadequate.
Moreover, the newly developed methods and the organoid model are in keeping with the overall trend in the life sciences and pharmaceutical industry toward personalized medicine. For instance, Cowan considers that, in the future, a patient suffering from a rare eye condition might well have an organoid grown in a laboratory from cellular material taken from their own body. Such organoids would make it possible to test alternative treatments, with the most promising option then chosen for the patient. In addition, thousands of active ingredients could be serially tested on thousands of organoids with no need for donor organs, and the probability of the results being transferable to humans would be far higher than if the mouse model were used.
More articles in the current issue of UNI NOVA.