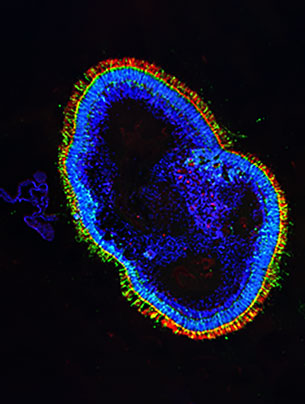
New retinal tissue from skin samples.
Text: Christoph Dieffenbacher
To better understand certain eye diseases, researchers are recreating human retinas in the lab. To accomplish this, they create tissue cultures known as organoids from human skin and blood cells.
The young Austrian molecular biologist Magdalena Renner specializes in organoids – tiny organs comprising a small number of cells artificially grown in culture from reprogrammed stem cells. For her doctoral research in Vienna, Renner worked with brain organoids, creating miniature brains from a handful of cells and studying how they develop.
Now, Renner leads a team at the Institute of Molecular and Clinical Ophthalmology Basel (IOB) devoted to retinal organoids. The goal of her work, which straddles basic and applied research, is to better understand various eye diseases. Her team has already succeeded in engineering relatively highly organized forms of human retinal tissue in the lab.
Complex nerve tissue
The retina, a multi-layered membrane lining the inside wall of the eye, is anything but a simple organ. It is a highly complex tissue structure “with a variety of cell types performing a variety of functions, arranged in no less than five different layers,” explains Renner. Or, to be precise, three layers of cell bodies and two intermediate layers that contain synapses among the nerve cells. In organoids, as in the human retina, photoreceptors are located in the outer layer. In the human body, photoreceptors capture incoming light signals and transmit them to the brain in the form of neural impulses.
This complex structure also makes the retina (Latin for “net”) highly sensitive – it is where most eye diseases originate. The most common diseases, some of which are hereditary, include age-related macular degeneration, diabetic retinopathy, vascular occlusions and retinal detachment. Renner highlights the retina’s fragility with an example: “failure of a single gene in the photoreceptors is enough to make a person blind.” Accordingly, many researchers believe that modeling the development of the retina and possible genetic mutations associated with certain diseases in the laboratory would be a major step toward new treatments.
“Our retinal organoids resemble three-dimensional miniature organs with an appearance and structure very close to those of actual retinas,” the researcher explains in her lab. The tiny structures only reach a size of around 2 by 2 millimeters before they stop growing. They contain similar cell types with functions that are related or identical to those of fully fledged retinal tissue. Renner explains: “We generally begin with a donor cell from a small skin biopsy, or – a less invasive option – a blood sample. Donor cells are reprogrammed into what are known as induced pluripotent stem cells using a method that has only been known for around twelve years.” These stem cells, which can differentiate into any kind of body cell, are then encouraged to multiply.
The eye as part of the brain
Renner recalls how much the early results of her research surprised even her: “The process that the stem cells go through in the laboratory culture very closely mirrors the natural development of retinal cells in human embryos.” To begin with, the retinal cells develop as general brain cells, before subsequently specializing. “Obviously,” Renner remarks. “The eye is in fact a part of the brain.” At various points during the development process, the researchers separated organoids into individual cells, which they then dissolved in order to sequence the RNA of 60,000 individual cells. This enabled them to determine which genes were expressed in the various cell types. Their goal is to come as close as possible to mature retinal cells.
Aside from the cell cultures, Renner’s team also studies retinas from organ donors, analyzing them in terms of gene expression and comparing them against the lab-grown organoids. They found that a majority of the known genes associated with inherited retinal degeneration are expressed by particular cell types. “This is an important finding, as we want to treat the cells immediately affected by a mutation first,” Renner explains.
How difficult is it to recreate human retinas in culture? The main problem, according to Renner, is that the maturation process takes over 30 weeks – a huge investment in terms of time and effort. It also means the team has to plan its experiments a very long time in advance – and exercise a great deal of patience. One as-yet unsolved problem is that not all cells of the same type result in viable organoids.
Mouse model a poor substitute
In any case, there is still a long way to go before it will be possible to repair parts of the retina with custom-grown tissue, or cure eye diseases in humans. Unlike the cornea, the retina cannot simply be transplanted. Researchers are therefore exploring a different approach, in which cells operating defectively due to a hereditary mutation are supplied with a correct copy of the gene to restore their functionality. This is achieved by packaging the appropriate sections of the retinal gene into viruses that are then injected into the ocular fundus – a therapeutic approach that is very easy to test in organoids. According to Renner, this method is promising as the eye is largely overlooked by the immune system, meaning it will not immediately seek to fight off the intruding virus.
Possible applications of this method or other therapies resulting from organoid research would still have to be tested on animals, Renner points out. The mouse model widely used in eye research laboratories, however, has a major drawback in this regard: “Mice have very poor eyesight, as their retina is built quite differently to that of humans.” At the center of our retina is the fovea, a region equipped with special photoreceptors that play a key role in color recognition and focus. Mice lack this region. Aside from humans, the fovea is only found in a few primate species, so it is unlikely that tests on animals can be eliminated altogether, Renner says. Nevertheless, she hopes that most of the necessary testing can one day be performed on organoids instead.
More articles in the current issue of UNI NOVA.