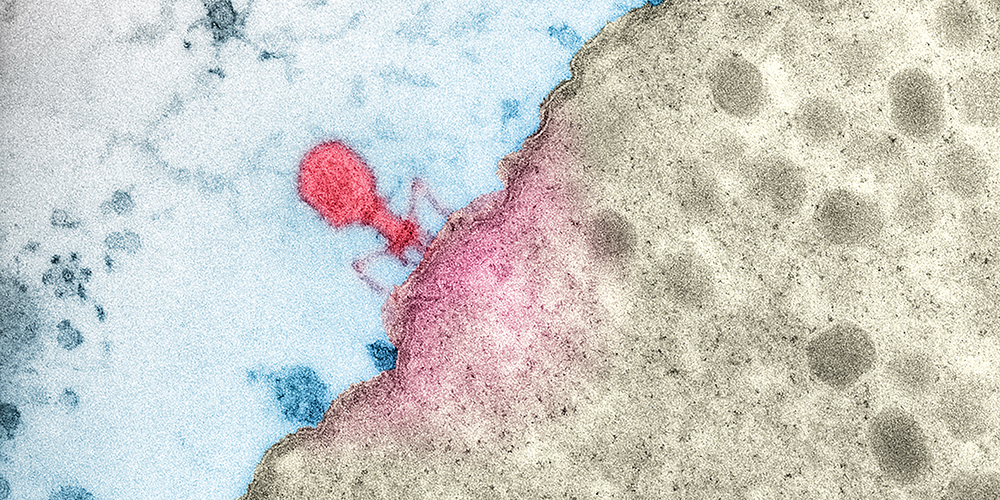
Combating bacteria with viruses.
Text: Yvonne Vahlensieck
As viruses that specialize in attacking bacteria, phages promise huge potential for the treatment of infections. Scientists in Basel are searching the natural world for new species of phages that can help them identify vulnerabilities in bacteria.
When the microbiologist Dr. Alexander Harms from the Biozentrum at the University of Basel needs new materials for his research, he simply goes down to the Rhine, a sewage treatment plant or a nearby park. There, he collects samples that are teeming with the subject of his experiments. These bacteriophages are viruses that penetrate bacterial cells and multiply inside them, killing the bacteria in the process. As such, they are considered a new wonder weapon in the fight against infections – especially as a growing number of pathogens are developing resistance to antibiotics.
A return to old ideas
Actually, this is not an entirely new approach. Around 100 years ago, ingenious doctors successfully used bacteriophages to combat bacterial infections. With the discovery of antibiotics, however, the method soon vanished into obscurity in most places. Phages continued to serve as an important model system for molecular biology research before finally falling out of use altogether in the 1980s. “Whereas research has advanced steadily in other areas, phage research has skipped a generation,” explains Harms. He finds this extremely regrettable, especially considering that the Biozentrum was once a world leader in this area. Indeed, the Basel-based professor of microbiology Werner Arber received the Nobel Prize in 1978 for research projects in which phages played a vital role.
In light of the antibiotic crisis, interest in phage research has seen a renaissance in recent years. “Thanks to a whole host of new techniques, such as rapid genome sequencing, researchers have realized just how many unknown species of phages are out there waiting to be discovered,” says Harms. Every drop of water and every speck of soil contains thousands upon thousands of phages – including, in all likelihood, some with the potential to neutralize dangerous bacteria using mechanisms that are still unknown to science.
Targeting dormant bacteria
This precisely is the basic premise of Harms’ research project: From his samples, he isolates phages that can kill off pathogens such as Salmonella, E. coli or Staphylococcus. Then, he selects the most promising specimens for more rigorous analysis using the latest techniques in molecularbiology, as well as many of the classical methods that the Biozentrum helped develop decades earlier. His aim: to determine what tricks the phages use to eliminate the bacteria and which genes are involved.
Harms is particularly interested in bacteria that evade treatment with antibiotics by temporarily lapsing into a sort of deep sleep. These so-called persister cells are thought to be responsible for chronic infections such as cystitis and pneumonia in patients suffering from cystic fibrosis. Despite repeated courses of antibiotics, these chronic infections never fully disappear. Rather, they flare up time after time and can even prove fatal in the worst cases. With this in mind, Harms is specifically looking for phages that can attack bacteria in their dormant state: “We then want to identify the genes that help phages destroy persister cells.”
Aiming to construct new phages
This systematic approach is very different from classical phage therapy, which has recently been the subject of frequent media coverage. In the classical method, physicians scour water samples from sewage treatment plants, for example, for a phage that just happens to attack the precise bacterium with which a patient is infected. After being multiplied, this phage is often administered to the patient under time pressure without thorough analysis of its mode of action. Harms has concerns about this approach: “The problem is that everything is very empirical. You don’t know exactly what’s going on between the phages and bacteria in the patient’s body or why it works in some cases but not in others.”
The researcher therefore plans to gradually amass a whole arsenal of phage genes and to use these to build tailor-made phages based on a number of well-known basic types. For example, the phages could be specifically geared toward combating chronic diseases, in which case they would benefit not only single individuals but also larger groups of patients suffering from the disease. Harms also hopes that the insights derived from his analyses will feed into the targeted development of other antibacterial drugs.
No problems with resistance
The use of phages offers another key advantage over antibiotic therapy. Experts believe there is little risk of bacteria developing lasting resistance to phages, and even if the bacteria develop mechanisms to defend themselves, the phages will adapt to these mechanisms very quickly. After all, they’ve been one step ahead in this evolutionary arms race for millions of years. “Bacteria evolve very quickly. But there’s something that evolves even faster, and that’s phages,” says Harms. He would also like to arm his custom-built phages to attack bacteria at several vulnerable sites at once, reducing the risk that bacteria could escape the phages by means of a simple adaptation. There’s also little chance of dangerous side effects, as experience has so far shown phages to be harmless to humans.
Like many researchers around the globe, Harms is confident that there are numerous potential discoveries lurking in the world of phages – a world that a group of high-school students had the chance to discover for themselves this summer as part of the “Basel Summer Science Academy.” After collecting samples from the Rhine, they isolated phages that Harms now plans to characterize in greater detail. There’s a chance that one of these specimens will contain an undiscovered gene – which may one day help restore patients with serious infections to full health.
More articles in the current issue of UNI NOVA.