New neurons for the brain.
Text: Yvonne Vahlensieck
New neurons are made by stem cells located in the adult mammalian brain. Much remains to be understood about this process, known as neurogenesis – but there are hopes that one day it could be harnessed to promote brain repair.
Throughout our entire life, our bodies are fighting decay: specialized stem cells continuously supply new cells for our blood, skin, and internal organs. For a long time, however, neuroscientists believed that this renewal of cells was not possible in the brain. “An adult brain does not make new neurons” was dogma for a century.
Then a revolution in neurobiology overthrew the old model. Around 50 years ago, evidence emerged that new neurons can in fact be made in the adult brain. Since then, stem cells located in specific areas of the brain have been found to be behind this process. Nevertheless, a great deal remains to be understood, such as how these new neurons participate in brain function and whether stem cell growth can be stimulated by external factors. It is hoped that finding the answers to these questions might one day lead to stem cells being used to repair brain damage.
Migrating cells
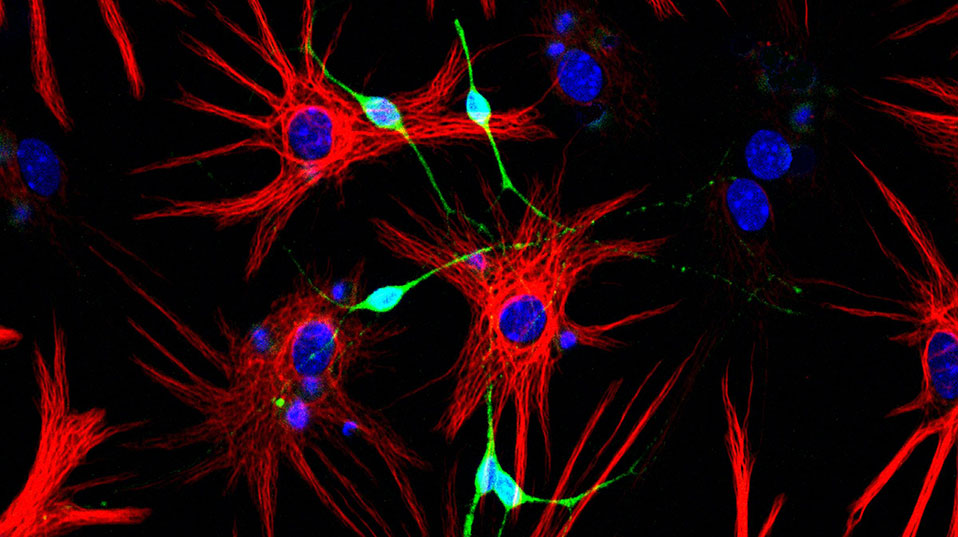
One of the two areas of the brain in which researchers have found stem cells, the lateral ventricles, are the focus of research carried out by Fiona Doetsch at the University of Basel’s Biozentrum: “Such stem cell niches are highly complex,” explains the Professor of Molecular Stem Cell Biology. The niches provide a microenvironment that fosters and nourishes stem cells. It is also here that they receive the signals telling them to diff erentiate into specific cell types and then migrate to other areas of the brain. Several years ago, Doetsch and her team showed that the stem cells in the lateral ventricles are a type of glial cell: “Since then we have been trying to understand how these stem cells are maintained and what activates them.”
One of the main tasks of the stem cells in the lateral ventricles is to make neurons for the olfactory bulb, a brain region crucial to our sense of smell. “In mice approximately 30,000 new neurons migrate daily from the lateral ventricles to the olfactory bulb,” Doetsch explains. To this end, the cells form long chains and flow through a tunnel made up of glial cells. Upon arrival in the olfactory bulb, the new neurons integrate into existing circuits, where they help transmit odor signals captured by the nose to the brain.
Which factors affect the activity of stem cells is only partly understood so far. However, research has shown that the formation of new neurons is suppressed by stress and boosted by physical exercise, for example. “This result reveals that external stimuli can also impact the generation of neurons,” says Doetsch.
A mosaic of stem cells
In an effort to identify these stimuli, the Canadian born stem cell biologist has undertaken a detailed investigation of the stem cell niche. Initially, all that was known was that stem cells are located in a thin layer near the ventricle walls. Now it is clear that the stem cells are arranged in such a way that on one side, an extension of the cell known as a process touches the cerebrospinal fluid which flows through the ventricles. On the other side, a process contacts blood vessels. Furthermore, the stem cells are connected to neurons from other brain areas. This means that stem cells receive and process information about the state of the organism from multiple sources.
The latest results from Doetsch’s laboratory highlight the complexity of the stem cell niche: The stem cells located there are in different states, forming a mosaic of activated and dormant cells. What is more, their location inside the niche provides information about the type of cell they will differentiate into after activation. “But it is still not known whether the same pool of cells is giving rise to new neurons under normal conditions as for repair of brain damage.”
Key role for neurotransmitters
This investigation into the niche’s structure revealedthe sources that stem cells receive signals from, but identifying the molecules involved required different experimental approaches. “We have tried for manyyears to activate the stem cells,” recalls Doetsch. But it was not until she tested a library of over 1,000 known biologically active compounds that she succeeded: It emerged that certain neurotransmitters – the chemical messengers of the brain – play a crucial part in activating stem cells. One of these transmitters was beta-endorphin, which in other contexts is released by the brain in response to pain or stress, for example.
This result led Doetsch and her team to the discovery that beta-endorphin-producing neurons in the hypothalamus are in contact with a highly specific subset of stem cells via long-range fi bers, regulating the formation of a particular subtype of olfactory bulb neurons. The division of this subset of stem cells is also infl uenced by diff erent feeding patterns such as fasting, via the activity of these hypothalamic neurons. Accordingly, it is possible that the stem cells could help mice adapt to variations in food supply. These findings confirmed that certain factors can stimulate the production of diff erent types of neurons by stem cells depending on the situation and environmental conditions.
Towards brain repair?
The extent to which these findings, obtained primarily from experiments in mice, can be applied to humans, is currently unclear. There is no question that stem cells are also present in the human brain – so in principle the potential for neuron renewal exists. But the degree to which it actually occurs in humans is currently a point of great contention among neuroscientists.
Nevertheless, researchers like Fiona Doetsch hope that they will one day be able to decipher the signals that regulate stem cells. This could make it possible to activate them in a controlled manner and direct them to specifi c brain areas. Brain damage resulting from, say, a stroke or Alzheimer’s disease would then no longer be irreparable, but curable.
Fiona Doetsch is Professor of Molecular Stem Cell Biology at Basel University’s Biozentrum. Her research focuses on stem cells located in adult mammalian brains.
More articles in the current issue of UNI NOVA.