Combating muscular atrophy.
Text: Martin Hicklin
Even in older individuals, strength training helps to combat the loss of muscle mass. Known as sarcopenia, this “curse of old age” is the focus of a research project at the Biozentrum.
It can affect people as young as 50, and anyone who has reached 70 really should work against it. Bit by bit, it chips away at their strength and mobility, and the underlying causes are anything but clear. This insidious condition is known as sarcopenia, from the Greek sarx, flesh, and penia, poverty – and is considered the “curse of old age”. Muscle mass is lost, and it becomes difficult to build it back in older age. The handshake becomes weaker, brisk walking becomes impossible, and falls become more frequent – often with disastrous consequences. If an effective way could be found to combat this process, it would be invaluable in more ways than one.
Disrupted equilibrium
Why the equilibrium between muscle build-up and breakdown deteriorates – and what exactly underlies these processes at the molecular level – is largely unknown, says Professor Markus Rüegg, a neurobiologist at the Biozentrum of the University of Basel. This gap in knowledge is all the more worrying given the growing number of susceptible older people in the population – and the serious consequences that this entails.
It made good sense, therefore, for researchers at the Biozentrum to set up a project focusing on sarcopenia, pooling their existing forces in order to investigate which regulatory systems are malfunctioning or no longer responding to signals. Rüegg has teamed up with Professor Christoph Handschin and the systems biologist and bioinformatician Professor Mihaela Zavolan. Research by multiple groups on the same topic has already proven to be valuable. This particular collaboration is funded by the Swiss National Science Foundation as part of the “Sinergia” program, and researchers who work on aging from Novartis are also participating in the project.
The necessary synergies are in place: Rüegg and his group study the processes that take place between nerves and muscles. They’re also seeking therapeutic approaches to rare muscular diseases such as dystrophy, in which the signaling pathways and muscle build-up and breakdown are disrupted. This work also offers insights into muscular atrophy in old age. Handschin and his group investigate what happens to healthy muscles during training – surprisingly, a field in which many areas still remain unexplored. Handschin has also been studying a protein known as PGC-1α, which supports muscle function and connections to nerves by building up mitochondria, the so-called powerhouses of cells. Finally, Zavolan knows how to handle the large datasets that are collected during the experiments.
The control center inside cells
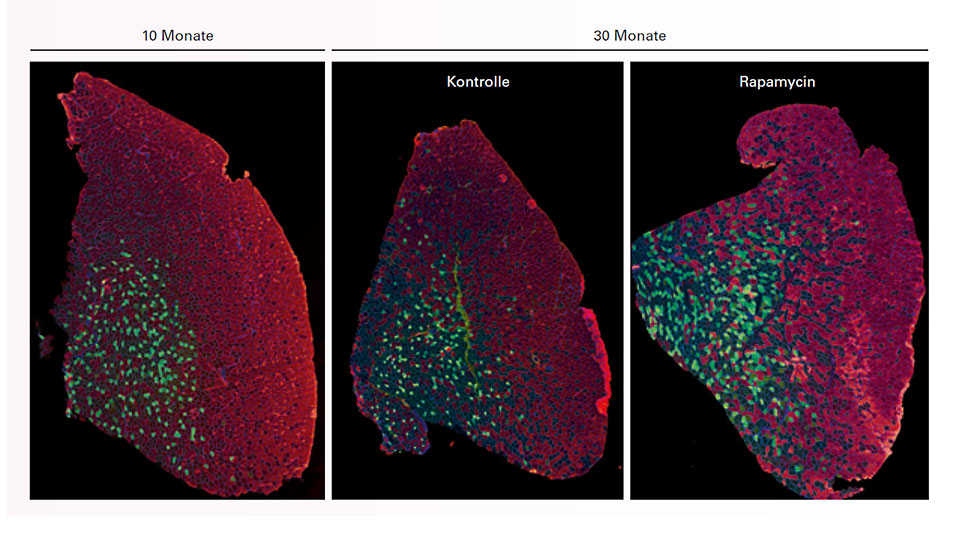
The Biozentrum also has another ace up its sleeve. Its researchers are well acquainted with a central control center inside the cell, which also plays a key role in the function and maintenance of muscles. It goes by the name of mTORC1 – short for mammalian target of rapamycin complex 1 – and was discovered last century by the award-winning biochemist Professor Michael N. Hall, who is also a researcher at the Biozentrum. “I’ve already collaborated with him in the past,” says Rüegg.
mTORC1 remains one of the focuses of the sarcopenia project. This cellular controller detects the availability of nutrients by responding to the protein building blocks (amino acids), as well as to the energy status of a cell and other factors needed for building proteins. If something is missing, the mTOR complex inhibits the production of proteins, including those destined for the muscles. “We know at least that mTORC1 can impede muscle build-up and accelerate the onset of sarcopenia,” says Rüegg. Rapamycin and other “rapalogs” with a similar structure inhibit mTORC1. However, if the switch – mTORC1 – is turned on, because all of the components are present, proteins needed for cellular life are produced. This is the mechanism by which mTORC1 determines cell size and growth.
Fasting prolongs lifespan
Conversely, a process known as autophagy (self-eating) is accelerated, ensuring that defective proteins with incorrect folding are degraded and that their useful components become available again – like on a sort of spare part exchange. Fasting and jogging cause the body to switch over to this process, which might be why some people say they feel better after fasting. They actually are in tidier shape. After just 24 hours of hunger, a mouse begins to break down its muscles and restructure its body. Fasting – also referred to as calorie restriction – prolongs the lives of mice (and monkeys).
Autophagy is an important homeostatic process that can also break down. It may be that some things become less effective with increasing age, something or other just stops and then gets in the way. The “Sinergia” project in Basel has set out to better understand these processes, which are influenced by numerous factors. A series of mouse models – including from Novartis – are now either available or under development, allowing researchers to investigate the role of each factor.
Helping to improve quality of life
This work is not without its surprises. Indeed, it has already delivered results that run counter to expectations. For example, the permanent activation of mTORC1 in a mouse does not simply result in bigger muscles, but can actually cost the mouse its life. It may also be that, in old age, mTORC1 is constantly set to a slightly higher level of activation, and that it might be sensible to exert a dampening influence. This will become clear in a subsequent stage of the research, which is expected to produce the first generally applicable findings. “Presumably, the best solution would be a balance between stimulation and inhibition,” says Rüegg.
What is certain – and indeed proven – is that strength training has benefits and triggers protein synthesis even in older people. The signal pathways that are switched on in our early years are still present in old age. They are just less efficient. “We’re currently analyzing molecular data and will then proceed with new models based on the results,” Rüegg says of the prospects for future research. In any case, this remains a hot topic in science. There is great hope that advances in modern technology will also pave the way for substantial improvements in quality of life until well into old age.
Markus Rüegg is Professor of Neurobiology at Basel University’s Biozentrum. His research interests include neuromuscular disorders and muscle atrophy in general.
Weitere Artikel in der aktuellen Ausgabe von UNI NOVA.