Shapeshifting gates guard the cell nucleus
An international study led by the University of Basel has discovered that nuclear pore complexes – tiny gateways in the nuclear membrane – are not rigid or gel-like as once thought. Their interiors are dynamically organized, constantly moving and rearranging. The findings reshape our understanding of a vital transport process in cells and have implications for diseases and potential therapies.
03 December 2025 | Christine Möller
Imagine the cell’s nucleus as a bank vault protected by a highly sophisticated security system: the nuclear pore complex (NPC). Only proteins carrying the correct “key” – specialized transport factors – are granted exclusive access. This selective control over what enters and exits the nucleus is essential for ensuring proper communication between the genome protected inside it and the cellular machinery outside.
Nanoscience leads to new biological insights
Despite its importance, the NPC’s inner workings have remained a mystery. Its transport channel is lined with highly flexible protein “threads” – the FG nucleoporins (FG Nups) – that create a selective barrier whose ultra-fine organization has eluded even the most powerful electron microscopes. Because the FG Nups can form gel-like assemblies outside of cells, older models have compared the NPC’s function to a rigid sieve.
Now, a team led by Argovia Professor for Nanobiology Roderick Lim from the Biozentrum and the Swiss Nanoscience Institute, University of Basel, has used high-speed atomic force microscopy (HS-AFM) to film never-seen-before nanometer scale movements with millisecond resolution directly inside the pore. The discovery of this extraordinary shape-shifting behavior within NPCs has now been published in Nature Cell Biology.
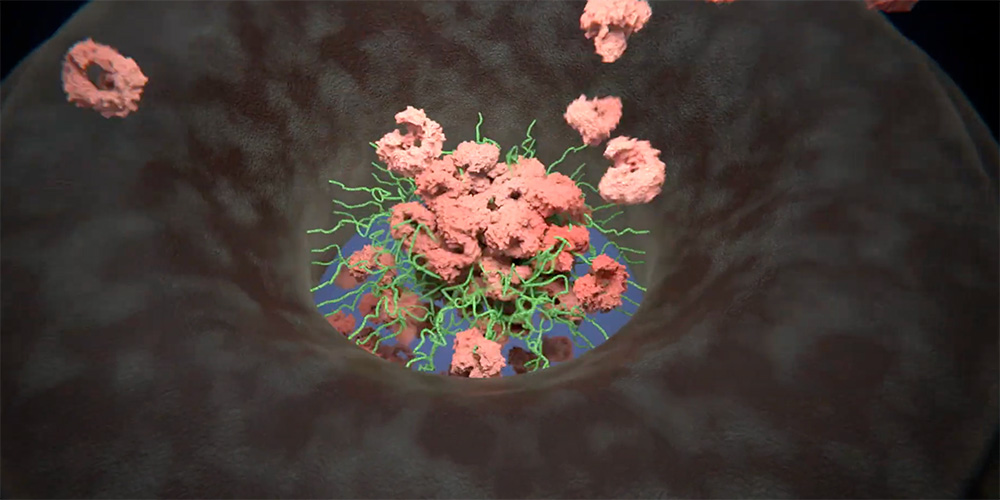
Model of the nuclear pore complex transport barrier
Tethered within the pore are highly dynamic protein threads termed FG Nups (green). Under living conditions, cargo-carrying transport factors (pink) interact with the FG Nups, loosely forming a central plug that helps organize a dynamic transport barrier. Selective transport may proceed preferentially through the surrounding region. For clarity, cargoes are omitted and FG Nup density is reduced. (Animation: Enrique Sahagun, Scixel)
“The NPC barrier is loosely organized by a mobile central plug, whose identity has long been enigmatic. It turns out to consist of a dynamic mixture of transport factors, cargo molecules, and FG Nups that comingle along the pore’s central axis. This creates a highly adaptable system that reinforces the barrier while ensuring fast selective transport”, says Lim.
Go with the flow
The team uncovered this dynamic organization while studying NPCs from yeast cells. High-speed AFM movies also revealed highly fluid FG Nup movements that “radiated” towards the central plug inside the pore.
“After prolonged incubation, the central plug of NPCs disappeared but was restored by adding transport factors,” reports Dr. Toshiya Kozai, first author of the study. Remarkably, the transport factors also replicated NPC-like barrier function in artificial nanopores, demonstrating the generality of this behavior.
Hydrogels resemble sponges with holes
NPCs have often been compared to hydrogels. “This is because FG Nups form hydrogels in vitro – in a test tube – but these assemblies are thousands of times larger than NPCs. Plus, they consist of tangled fiber-like structures that are simply too big to fit inside an NPC, let alone the entire hydrogel body itself,” explains Lim. “When we examined them more closely, we found that the hydrogels were riddled with holes of irregular shapes and sizes – much like a kitchen sponge. Many of these holes were as large as NPCs or even larger, and could potentially mimic NPC-like behavior.”
The self-organizing, dynamic behavior revealed in this study offers a unified view of NPCs that aligns with long-standing structural and biochemical observations – with implications ranging from fundamental cell biology to the design of smart filters and drug delivery systems. Notably, restraining the pore’s dynamic state impeded selective transport into the nucleus, highlighting how essential such behavior is for the cell to function properly.
“The next challenge is to understand how cells fine-tune these remarkable nanomachines in response to changing needs – how the pores adjust to stress, regulate growth, and when they get jammed, contribute to disease,” adds Professor Michael Rout of Rockefeller University, who co-led the work.
The work is based on an international collaboration between researchers from the Biozentrum and the Swiss Nanoscience Institute at the University of Basel (Switzerland), Rockefeller University (New York, USA), Ikerbasque Foundation for Science (Bilbao, Spain), Biofisika Institute-CSIC-UPV/EHU-Fundacion Biofisika Bizkaia (Leioa, Spain), the University of Groningen (Netherlands), the Hebrew University of Jerusalem (Israel), the University of California, San Francisco (USA), Yale University (West Haven, USA), and Yale School of Medicine (New Haven, CT, USA).
Original publication
Kozai, T., Fernandez-Martinez, J., Kapinos, L.E. et al.
Karyopherins remodel the dynamic organization of the nuclear pore complex transport barrier.
Nature Cell Biology (2025), doi: 10.1038/s41556-025-01812-9